Vander waal Forces
"These are week attractive force between or among two or three molecules".
Origin of Vander wal attractive forces:
Due to mutul collision or some other factors and constant random moment of electron clouds around the nucleus get slightly distorted in one or some of the molecules :
1- Dipole - Dipole Interaction
2- Dipole - Induced Dipole Interaction
3 - Instentenous Dipole - Instentenous Induced Dipole
1- Dipole - Dipole Interaction
Dipole-dipole forces are attractive forces between the positive end of one polar molecule and the negative end of another polar molecule. Dipole-dipole forces have strengths that range from 5 kJ to 20 kJ per mole. They are much weaker than ionic or covalent bonds and have a significant effect only when the molecules involved are close together (touching or almost touching).The figures show two arrangements of polar iodine monochloride (ICl) molecules that give rise to dipole-dipole attractions.
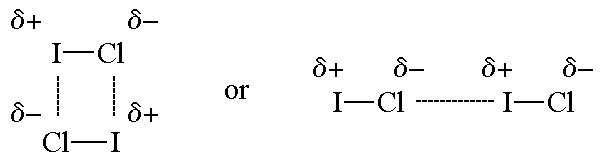
- Polar molecules have a partial negative end and a partial positive end.
- The partially positive end of a polar molecule is attracted to the partially negative end of another.
- In a ICl molecule the more electronegative chlorine atom bears the partial negative charge; the less electronegative iodine atom bears the partial positive charge.
- The partially positive iodine end of one ICl molecule is attracted to the partially negative chlorine end of another ICl molecule.
*A dashed line is used to represent an intermolecular attraction between molecules because these forces are NOT as strong as chemical bonds.
Physical Consequences of Dipole - Dipole Forces:
Both ICl and Br2 have the same number of atoms and approximately the same molecular weight, but ICl is a solid whereas Br2 is a liquid at 0oC. (Why?)
Answer: Intermolecular dipole-dipole attractions between ICl molecules are sufficient to cause them to form a solid at 0oC, whereas the intermolecular attractions between nonpolar Br2 molecules are not
2- Dipole - Induced Dipole Interaction
A dipole-induced dipole attraction is a weak attraction that results when a polar molecule induces a dipole in an atom or in a nonpolar molecule by disturbing the arrangement of electrons in the nonpolar species.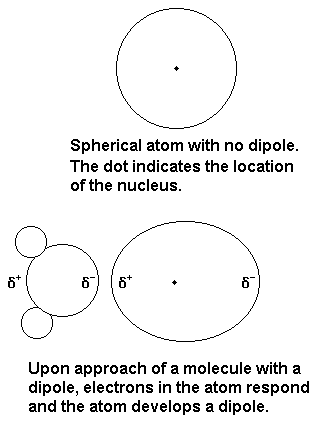
3 - Instentenous Dipole - Instentenous Induced Dipole
The temporary dipole in one molecule can induced a temporary dipole in near by molecule. As a result, the negative side of one molecule. is adjacent to the positive side of another molecule, because the dipole in the molecules are induced. This type of interactions between the molecules are called induced dipole - induced dipole interactions also known as London or Dispersion forces. E.g.
This molecule is perfectly symmetrical and tetrahedral in shape. Whilst there is polarity in each of the C-Cl covalent bonds, because of the symmetrical shape of the molecule, the molecule has no overall dipole.
References:
No comments:
Post a Comment