Structure and Hybridization:
How we can
understand this? “Easy” by solving question based on it.
SP
Example 1st: BeF2
Write
valence shell electronic configuration.
Be: 1s2,
2s2
1 - Write orbital configuration of valence shell only.
2 - Excite electron of 2s orbital into vacant p - orbital.
3 - 2s + 2p, having equal energy, now they are Hybrid orbital :)
4 - Hybrid orbital forms covalent bond with surrounded atoms such as F.
Thus, sp - Hybridization in BeF2
*What happen with 1s2 orbital electrons?
It remains as non-bonded electrons i.e. lone pair. Thus in this
example one lone pair is present in 1s orbital.
*What causes excitation of electron?
Presence of two F – atom causes this excitation.
*How F – atoms combine with this sp – Hybrid orbital?
C2H2 (sp - Hybridization)


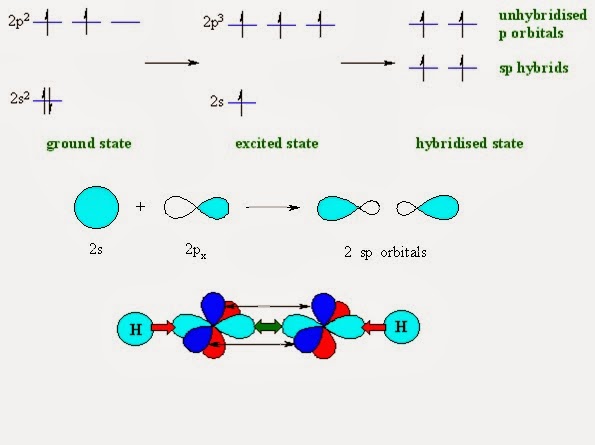

No comments:
Post a Comment